SolyPep is a fast and flexible random sequence generator for producing peptides selected for their aqueous solubility
The server first generates the required number peptides of desired length. This input library can alternatively be provided by the user in fasta format by pasting the sequences in the input field or by uploading a file. Each peptide sequence can be filtered according to a set of simple rules designed to guarantee the peptide solubility. The next step of the service will offer the processing of individual peptide sequences into 3D coordinates, files for use direct in autodock or vina can optionnally be added to the archive. It is important to note that the 3D generation step aims at providing starting conformations for flexible docking using a tool such as autodock, not predicting the conformation of the peptides in solution.Solypep online services are freely accessible for all users. Persons willing to have a private copy of the software should contact the authors.
Access the
SolyPep server @ the
RPBS Mobyle Portal.
When using this service, please cite the following references:
SolyPep: a fast generator of soluble peptides
submitted
[ Restricted to peptide of 5 to 15 residues ]
When using this service, please cite the following references:
SolyPep: a fast generator of soluble peptides
submitted
Overview
SolyPep has three different components implemented independently or grouped under the SolyPep service in the Mobyle platform.
-
Peptide sequence generation
The initial sequence of a given peptide is produced using a random drawing via a perl iterator. This ensures any of the 20 natural amino acids is properly selected and equally represented.
The peptide fasta sequence has the form:>pep-XXAA-UUIDwhere "XX" is the initial peptide size (5 to 15), UUID is a 32 bits identifier and SEQUENCE the output of the perl iterator. Example:
SEQUENCE
>pep-10AA-7e6fcf9690464a8f8f83bb5b7362080a
TSVEDESRAT
The sequence generation is limited to 1000 peptides per run.
Click here to access to the PepSeq standalone service. -
Peptide solubility filtering
The peptide solubility is assessed using a rule-based filtering applied to its sequence:
o discard if the first or the last amino acid is charged
o discard if the number of glycine or proline is more than one in the sequence
o discard if any amino acid represents more than 25% of the total sequence
o discard if the number of charged and/or of hydrophobic amino acids exceeds 45%
o discard if the number of gel-prone amino acids represents more than 75%
o discard if the absolute total peptide charge at pH 7 is more than +1
Click here to access to the PepSolubility standalone service. -
3D conformation generation
The three dimensionnal structure of the peptide of interest is generated using a fast or a more complete method.
The fast generation will provide a mathematical model of the peptide based on pre-calculated phi/psi angles for the peptide backbone, no optimization is performed on side-chains orientations.
MODELLER can be used to generate conformations of the peptide in helix, extended, and beta strand. This three dimensional conformation generation takes more time but will allow side-chains optimizations.
For peptides supposed to be cyclic -including two cysteines-, the MODELLER generation will automatically bridge the cysteines to produce a cyclic peptide. If more than two cysteines are found, the routine discards the peptide generation.
Click here to access to the PepBasic3D standalone service.
Usage
-
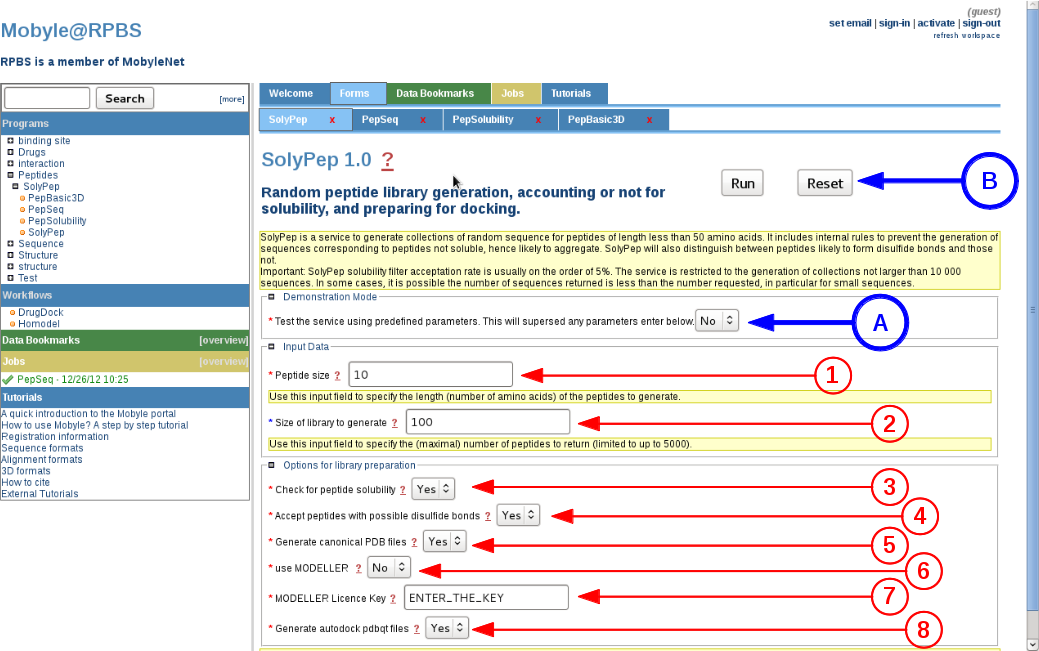
Demo mode (A)
By setting this option to "Yes", SolyPep will use pre-generated amino acids sequences to output the solubility validation, and the 3D conformation generation using the fast generation method. All other parameter values will be superseded. The aim of this demonstration mode is to illustrate the server functionalities, and together to provide the means for RPBS internal survey of its effectiveness. -
Peptide size (1)
The user has to provide the length of the peptide to generate. A classical input will be comprised of 7 to 15 amino acids. The lower and upper boundaries are set to 5 and 15 amino acids respectively. -
Number of peptide to generate (2)
The user asks for the final number of peptides to generate. The number is guaranteed except if the solubility criteria excludes more than 98% of peptides during the solubility validation. The maximum number for peptide generation is 1000 using the web server interface. -
Check for peptide solubility (3)
The main advantage of this server is to output a solubility prediction for each peptide. One user may wish to avoid this solubility filtering or may wish to use other validation tools for their specific peptide collection. If activated, the filtering will discard 80% to 95% of the randomly generated peptide sequences. -
Accept sequences with potential disulfide bridges (4)
Peptides containing two cysteines or more are prone to spontaneous cyclization, even if not synthesized as cyclic peptides. By default there are more prone to being discard during the solubility filtering so activating this option will force the inclusion of these peptides. To generate a cyclic peptide, please select the MODELLER conformation generator. -
Generate PDB files using the fast method (5)
The fast generation will provide a mathematical model of the peptide based on pre-calculated phi/psi angles for the peptide backbone, no optimization is performed on side-chains orientations. This method will generally provide 3D peptide conformations in a minute or so, depending on server load. -
Use MODELLER to generate PDB files (6)
If the user activate this longer generation method for producing the PDB file, it will have to input his own MODELLER key (7), as required by the MODELLER license terms. This conformation generation will allow a more complete optimization of the peptide backbone and of side chains conformations. This is also the recommended method to automatically generate cyclic if two cysteines are detected in the peptide sequence. A typical peptide run time will generally be one order of magnitude longer than the fast method. -
Generate PDBQT files for AUTODOCK and VINA (8)
Since peptides conformations can be evaluated as ligands for docking analysis, we provide pre-computed files using the AutoDock Tools as implemented in MGLTools. -
Launch the prediction (B)
Once completed, just activate the service using the "RUN" button (B).
Limitations
-
Amino acid sequence size
SolyPep prediction is limited to amino acid sequence of 5 to 15 residues, since we cannot guarantee the 3D conformation of a peptide longer than this, and exceptions of the "random coil" assumption already exists for peptides limited to this size. -
Peptide solubility
SolyPep is designed to guarantee the peptide solubility in water. It has a high rejection rate so perfectly soluble peptides may be discarded. On the experimental test set used for validation, no peptide declared "not soluble" proved to be easily soluble in water. -
Peptide conformations
PDB conformations generated using the fast method or the mode computationnally demanding MODELLER rendering is far from being optimal. The user should not use blindly the resulting structures as a basis for structural validations.
These sub-optimal conformations are intended to be used for further docking experiments where the algorithm should optimally modify any starting conformation to obtain the same pose. This will allow an indirect evaluation of the docking calculation since the pose should not be dependent of the initial input conformation.
Results
Each individual tool is presented below to illustrate the individual steps of the server, they are regrouped in one form directly in the integrated SolyPep Mobyle page.
Peptide generation
-
Sequences generation summary
After the peptide sequence generation, a synthetic graphical view is presented to the user in the form of a sequence logo. This allows a rapid overview of the amino acids statistics.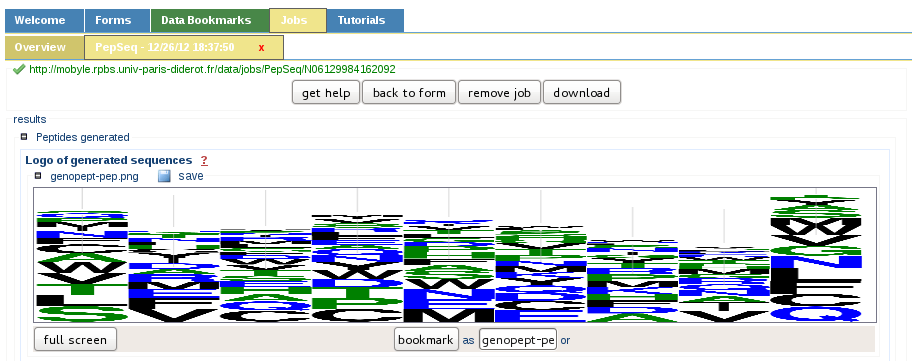
-
Information provided to the user
The sequences are provided as fasta files and potential cyclic peptides containing at least two cysteines are separated from the other peptides. If the user want to stop the prediction at this step, he can directly download the fasta files from the web interface.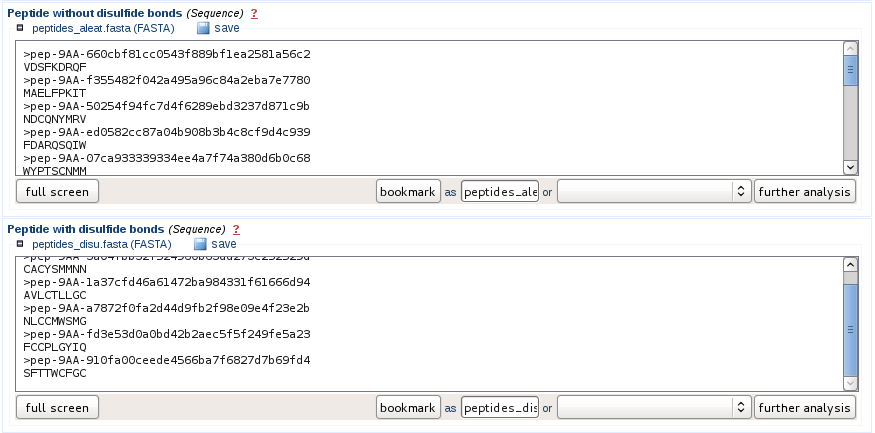
-
Sequence generation and filtering statistics
If the solubility filter has been applied, the statistics indicate how many sequences were generated in order to comply with the user needs. In the example below, 590 peptide sequences were generated to obtain 50 peptides validated as soluble.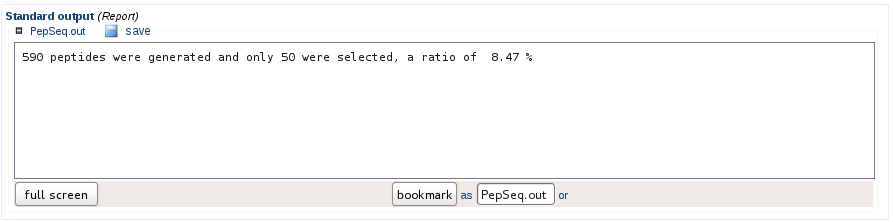
-
Another example of the sequence filtering is:
23 peptides were generated and only 3 were selected, a ratio of 13.64%.
Peptide solubility assessment
-
Check for solubility
By default the peptide sequence generation will discard peptides considered as not soluble, if you wish to conserve these sequences, the peptide solubility will be assessed but will be ignored for further processing. The user is informed about the sequences considered soluble or not.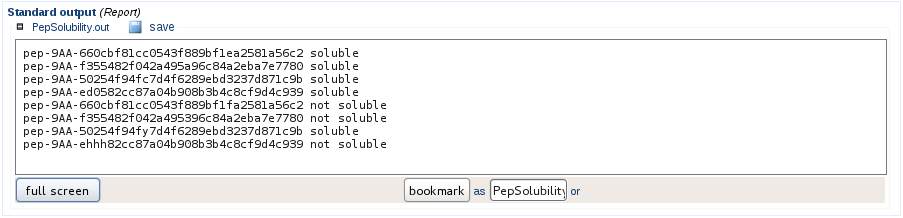
-
Input format for user-provided peptide sequences
The user-provided input sequences must be in FASTA format. The query peptide sequence must contain a string of only the 20 standard amino acids in uppercase, using the 1 letter code (see the pre-configured test example). The size of the input sequence can be no longer than 15 amino acids. -
Solubility validation performance
Usually, SolyPep solubility prediction for a given collection of peptides is performed within a minute.
Three dimensional conformations generation
-
Fast 3D conformations generation
The fast generation will provide a mathematical model of the peptide based on pre-calculated phi/psi angles for the peptide backbone, no optimization is performed on side-chains orientations. This method will generally provide 3D peptide conformations in a minute or so, depending on server load. -
3D conformations generated using MODELLER
Each peptide sequence will be converted into 3D coordinates using pre-defined regular conformations as implemented in the MODELLER routines (alpha helix, beta strand, and unspecified). The user can select the previously generated list of peptides as input using the selector or input his own list of peptides. The peptide conformation generation will take about one order of magnitude more time than the fast 3D conformations generator.
This simple step can easily be replaced by more elaborated routines such as implemented in PEP-Fold but each peptide generation will then need more than one hour instead of seconds using the sub-optimal generation of conformations from model reference structures as implemented in MODELLER.
-
Files prepared for docking experiments
Additionnally to the PDB files generated using the fast method or the MODELLER routines, the user can ask for a PDBQT generation. This ligand file is generated using the routines embedded in MGLtools. This allows a direct usage of the provided files so one user can directly benefit from our server to study their target for peptide binding. -
Additional services are available in Mobyle
Since mobyle is a platform providing independent services which can be chained, any step described here can be branched to another tool so the user can get additionnal informations, to address his own needs.
For instance instead of using Basic3Dbuilder or MODELLER for generating the conformation of peptides (one peptide or a list of peptides), PEP-FOLD can be called interactively via the drop-box menu.
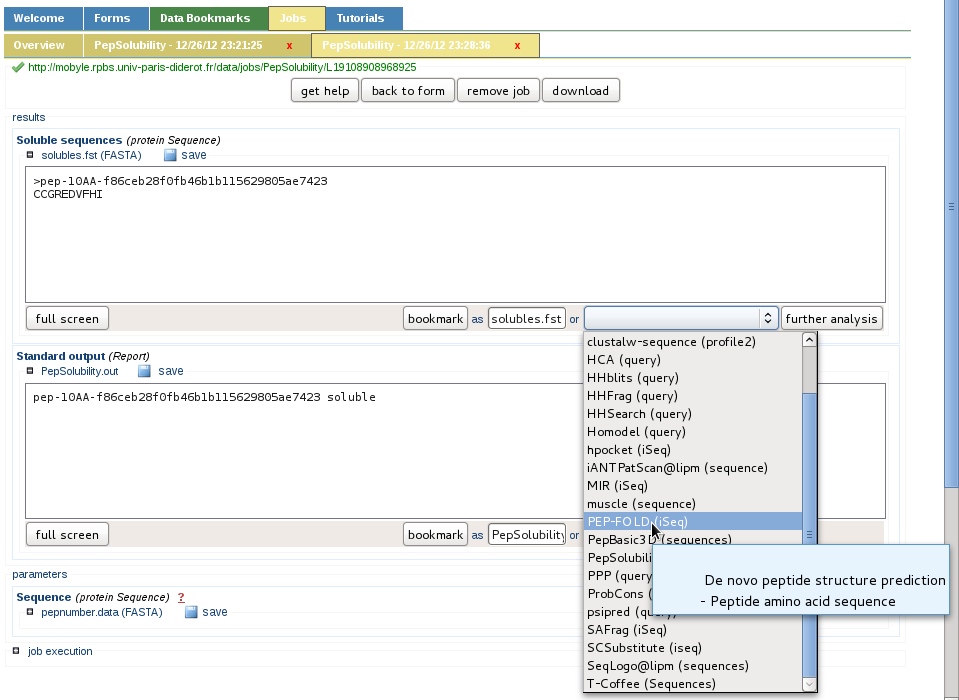 More services will be implemented in Mobyle if needed to the actual SolyPep service, or as independent components if the community asks for an improvement in this direction.
More services will be implemented in Mobyle if needed to the actual SolyPep service, or as independent components if the community asks for an improvement in this direction.
-
Data retrieval
Each peptide conformation is presented as a pdb file and and its accompanying pdbqt file ready for further analysis in docking experiments.
An archive link is provided to download all generated files in a central location.
Registered users will also receive the download link by email.
Validation of SolyPep predictions
SolyPep was designed with the aim to minimize the cost of synthesizing non soluble peptides. SolyPep has been used for the design of RANK antagonists ("De Novo Peptide Design of Novel RANK Antagonists: Theoretical Predictions and Experimental Validation." Teletchea et al., submitted."), which led to the synthesis of 58 peptides, where 41 were experimentally considered as “soluble in water” and 17 “non soluble”.
- All 17 “non soluble” peptides were correctly identified, i.e. no peptide experimentally validated as “not soluble” was wrongly attributed the “soluble” quality.
- SolyPep declared 14 peptides “soluble” over the 41 soluble peptides.
- According to these results SolyPep has an accuracy, i.e. the ability to predict correctly the status “soluble” or “not soluble” of a peptide, of 53%, a sensitivity, i.e. the ability to detect correctly soluble peptides, of 45%, and a very strong specificity of 100%.